Instrumentation
Beer's - Lambert's law is the fundamental law governing the absorbance of monochromatic radiation by homogeneous transparent systems. On the basis of these laws the technique employed for measurements by instruments called colorimetry and spectrophotometry.
Colorimetry
It is the simplest form of absorption analysis. The technique which is concerned with the determination of the concentration of a substance by measurement of the relative absorption of light with respect to a known concentration of the substance is called colorimetry.
Photoelectric colorimeter
The instruments which have a photoelectric device as detector instead of the eye and are used in the visible region are referred to as colorimeters. Filter is used to select the desired wavelength. These are known as a filter photometer or photoelectric photometer. The sensitivity of the instrument is considerably enhanced with the use of a photoelectric detector. Two types of photoelectric colorimeters are in use-
Single beam and 2) Double beam photoelectric colorimeters.
Single beam photoelectric colorimeter:
In modern instrument of colorimeter, the intensity of the transmitted radiation is measured by photoelectric cells. Such colorimeters are called photoelectric colorimeters.
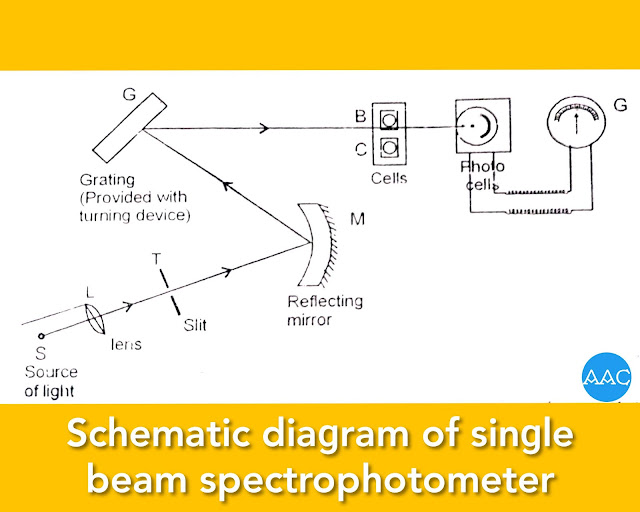
The essential components of single beam photoelectric colorimeter are
A source of light (S) (Tungsten lamp) held in a concave reflector. A collimating lens (L).
Galvanometer (G), An adjustable diaphragm (D)
A colored glass filter for monochromatising light (F)
The cuvette for holding the absorbing solution.(C). Barrier - type photocell (P).
Working:
The cuvette is first filled with an appropriate blank (solvent) and placed in the optical path. The incident light intensity is adjusted so as to obtain reading 100% transmittance or zero absorbance. After this adjustment, the cuvette is filled with the sample solution and placed in the optical path. It's percentage transmittance( %T) or absorbance (A) is measured. The concentration of the sample solution can be determined using Beer-Lambert's equation.
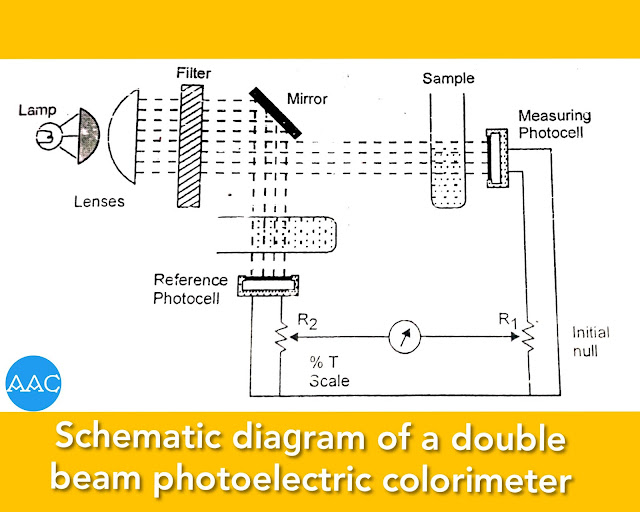
Double beam photoelectric colorimeter
In order to operate this instrument, the null balance galvanometer is adjusted mechanically to bring the needle at mid scale with the lamp OFF. Then, the lamp is switched ON and blank solution is kept in both light beams.
The potentiometer dial R₂ is adjusted to read 100% transmittance and then the slide-wire contact R₁ is adjusted to null the galvanometer. Standard and unknown solutions are introduced into the measurement beam and slide-wire contact R₂ is adjusted to renull the meter. The transmittances (linear) or absorbance (non-linear) can then be read out from the potentiometer dial for each sample.
Advantage of Double Beam Instruments
Although the double beam instruments are more complicated and expensive, they do offer the following advantage:
It is not necessary to continually replace the blank with the sample or to adjust zero absorbance at each wavelength as in single beam units.
The ratio of the powers of sample and reference beams is constantly obtained and used. Any error due to variation in the intensity of the source and fluctuation in the detector is minimized.
Because of the previous two factors the double beam system tends itself to rapid scanning over a wide wavelength region and to the use of a recorder or digital readout.
Spectrophotometry
Colorimetrically technique suffers from certain disadvantages like limited utility, low sensitivity etc, which have been removed in spectrophotometry. Development of this technique is discussed in details.
In a photoelectric colorimeter, a light filter has been placed after the source of white light. The radiations emerging from the filter have wavelengths within a small range known as wave band. The band width is about width 20 nm to 50nm.
If a light filter is replaced by a device known as monochromator, we can have a beam of radiations of particular wavelength i.e. a beam of monochromatic radiations. This has a band width of less than 1nm.
In filter photometer the bandwidth is more and due to this it has two disadvantages.
True absorption curve cannot be obtained.
Beer's law is not followed in true sense.
In order to remove these difficulties, in spectrophotometer meter, monochromator is used which may be prism of diffraction grating or an interference wedge together with two narrow slits. Any one of them is capable of isolating a much narrow band of wavelength (1nm).
The amount of light reaching the detector of spectrophotometer is generally much smaller than that available for a colorimeter, because of it the small spectral bandwidth. Therefore a more sensitive detector is required.
A photomultiplier or vacuum photo-cell is used instead of photometer.
As the technique is applicable to visible,UV and IR regions, different source of radiation are used.
Spectrophotometer
It is a combination of spectrometer and photometer. The function of spectrometer is to provide a beam of radiation of a selected wavelength and that of photometer is to measure the intensity of the transmitted radiation.
The essential parts of spectrophotometer are-
Source of radiation:
The source is used in a spectrophotometer are tungsten halogen lamp in visible region (380 to 780 nm), hydrogen discharge tube for UV region and deuterium lamp (190-375 nm) or Nernst Glower for IR region.
Monochromator:
Light from the radiation source is allowed to pass by means of a lens (L) through a narrow slight (T) and then reflecting mirror M on to an optical grating G or a prism, which divides light into narrow spectral regions corresponding to different wavelengths.
Absorption cell:
The light of a desired wavelength emerging from the grating is allowed to pass through the cuvette B containing solution under examination. For studies in visible region, the cell made up of corex glass is used while for UV region, a quartz cell is applied.
Detector:
The light coming out of the solution after absorption, is allowed to fall on the detector D. The role of detector is to respond to the intensity of radiation. In spectrophotometer generally photo multiplier tubes are used as detector.
Indication:
A galvanometer records the intensity of the light falling on the photoelectric cell.
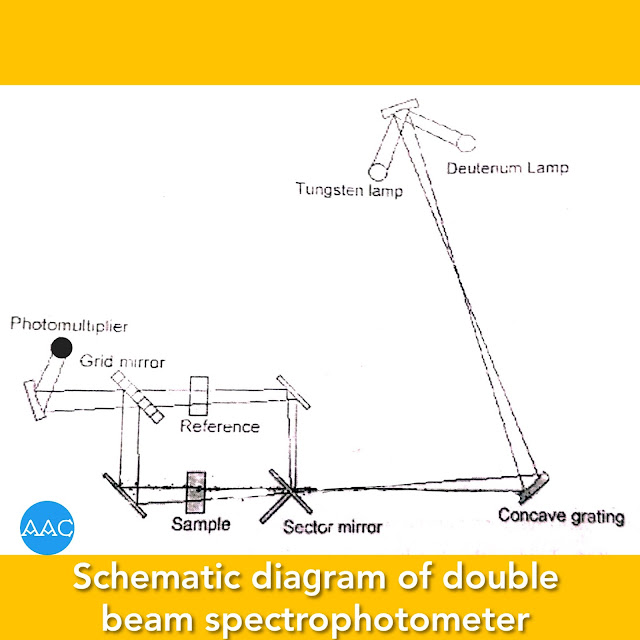
Double-beam spectrophotometer
Double-beam spectrophotometers has a beam splitter like chopper that splits the beam obtained from source into two components of equal intensity. One beam passes throughout the solvent while the other through sample cell. The difference is recorded by the photomultiplier tube. All the other components are similar to single beam instrument. Schematically, it can be shown as follows-
Application of Colorimetry and
spectrophotometry in quantitative
inorganic analysis.
Colorimetric and spectrophotometric techniques use Beer-Lambert's law as a basis for quantitative determination of a species in solution. In quantitative chemical analysis of any substance, following steps are involved.
Development of colour:
If the species to be analyzed colorimetrically is colourless, it is treated with suitable reagent that imparts colour to the solution. This reagent is added in excess of the species under study. Also, this reagent should not absorb at the same wavelength. Such colour development is not required in spectrophotometry, provided, the species absorbs in either visible or UV region.
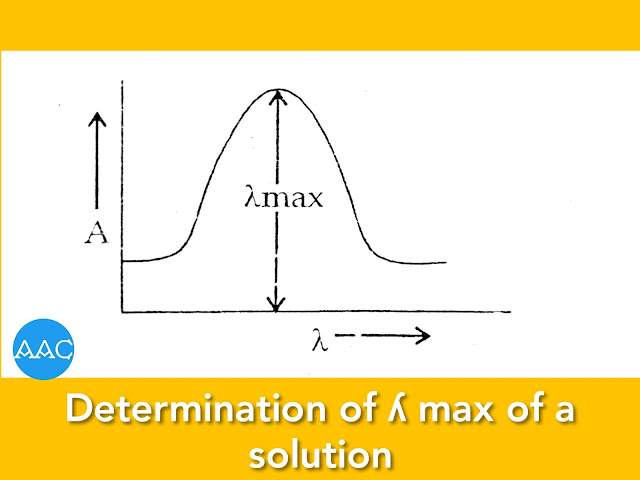
Determination of λ max:
The wavelength at which the substance absorbs maximum is called as λ max. At this wavelength, the value of molar absorptivity is maximum and hence the sensitivity is maximum. It is determined by recording absorbance of any of the standard solutions of the species under study at different wavelengths.
In case of colorimeter, it is not possible wavelength linearly. So, absorbance is recorded using different filters. A graph is plotted between absorbance and wavelength. Maxima of this graph corresponds to λ max (Figure).
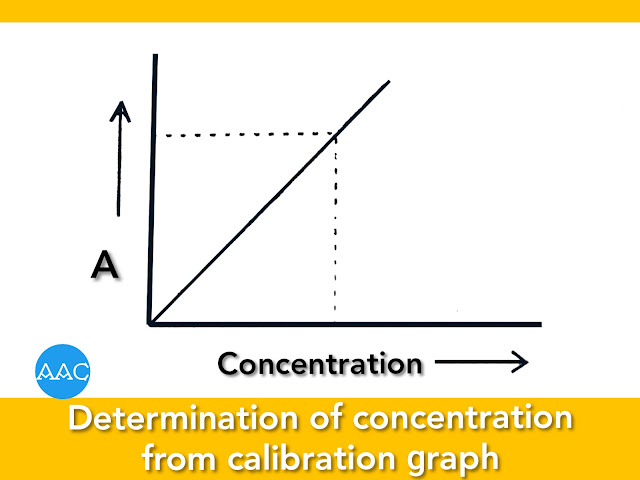
Preparation of calibration curve:
A series of solutions containing different concentrations of species under study is prepared and absorbances are recorded at wavelength selected previously. A graph is plotted between concentration of the species and absorbance. It is a straight line passing through origin and called as calibration graph.
Estimation of concentration of analyte:
Sample is treated in the similar manner as that of the series of standards. Colour is developed and absorbance e recorded at the wavelength. From calibration graph, concentration of the analyte can be determined as shown in figure.
Estimation of copper as copper-ammonia complex
Principle:
A series of standard solutions of copper sulphate penta hydrated is treated with ammonia to get blue cuprammonium complex, and is diluted to a definite volume. The absorbance of each of these solutions is measured at 590 nm since the complex shows maximum absorbance at this wavelength. The absorbance values are plotted against concentration to get a calibration curve.
The analysis involves following steps-
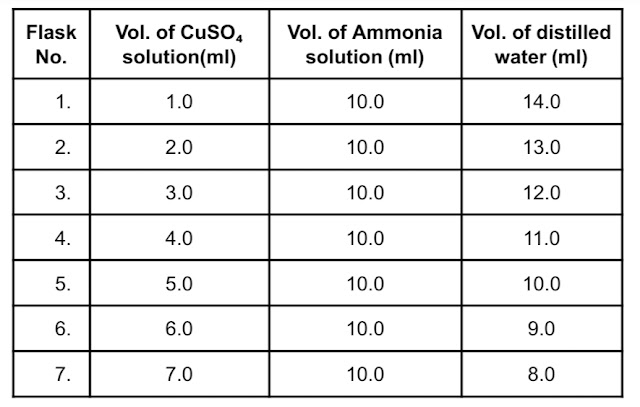
1. Preparation of standards:
A 0.01M copper sulphate solution is prepared in distilled water maintaining slightly acidic condition to avoid hydrolysis of copper sulphate. This can be achieved by adding a few drops of sulphuric acid solution. A series of standards is prepared by taking different volumes of CuSO₄ solution in 25 ml volumetric flasks.
2. Development of colour:
To each of the volumetric flasks, 10 ml of 1:1 ammonia is added to develop the colour and volume is made up of 25ml with distilled water.
3. Determination of λ max:
The wavelength at which the substance absorbs maximum is called as λ max. At this wavelength, the value of molar absorptivity is maximum and hence the sensitivity is maximum. It is determined by recording absorbance of any one of the above systems.
In case of colorimeter, it is not possible wavelength linearly. So, absorbance is recorded using different filters. A graph is plotted between absorbance and wavelength. Maxima of this graph corresponds to λ max (Figure). For copper ammonia complex, it is 590 nm.
4. Preparation of calibration curve:
Absorbances are recorded at wavelength selected previously. A graph is plotted between concentration of the species and absorbance. It is straight line passing through origin and called as calibration graph.
5. Estimation of concentration of analyte:
Sample is treated in the similar manner as that of the series of standards. Colour is developed and absorbance e recorded at the wavelength. From calibration graph, concentration of the analyte can be determined as shown in figure.
Difference between colorimeter
and spectrophotometer.
Colorimeter
In a photoelectric colorimeter colour filters are used to select desired wavelength.
The radiations emerging out from the filters have wavelength within a small range. (Band-width is 20 to 50 nm).
In colorimeter a photoelectric device used as detector is photocells.
They are used in only visible region.
Deviations from Beer-Lambert's law are observed.
Colorimeters are generally used for the quantitative analysis of coloured substances.
Spectrophotometer
In spectrophotometer, a mono-chromator is used to select desired wavelength.
The radiations emerging out from the monochromator have very narrow wavelength range. (Band width is <1nm.)
In spectrophotometer, photo multiplier tubes or high vacuum photo missive cells are used.
They are used in visible, UV and IR regions.
Beer-Lambert's law is perfectly obeyed.
Spectrophotometers are mostly used for the structure elucidation of colourless and coloured substances in addition to quantitative analysis.














0 comments:
Post a Comment