Liquid- liquid extraction is a technique in which a solution (usually aqueous) is brought in contact with a second solvent (usually organic), essentially immissible with the first, in order to bring about a transfer of one or more solutes into the second solvent. The separation are convenient, rapid and simple. Mostly, they are carried out in separatory funnel.
Principle
The technique of solvent extraction is based on Nernst's distribution law. According to this law "When a solute is equilibrated between two immiscible solvents in contact with each other, it gets distributed between the two solvents in fixed proportion ". The ratio of concentration of a solute in two solvents is called as distribution coefficient or partition coefficient. It is given by K=Co/Cw
Where,
Co= concentration of solute in organic phase
Cw= concentration of solute in aqueous phase
The species to be separated is converted into a form, which can be extracted by other solvent.
After conversion into suitable form, the solution is brought in contact with other solvent that is immiscible. It is equilibrated for sufficient time so that the species passes selectively into other solvent and can be analyzed subsequently.
e.g. If a metal is to be extracted, it is converted into complex and then extracted into organic solvent.
Technique
Solvent extraction is carried out in three steps.
Formation of distributable species:
If a metal ion is to be extracted from aqueous solution into organic solvent, it can not be directly brought in contact with organic liquid. Firstly, it is treated with suitable ligand to form a complex.
M + nL → MLn
Distribution:
After formation of distributable species, immiscible solvent is added to this solution and shaken vigorously in order to establish equilibrium the concentrations of organic and aqueous phases follow Nernst's distribution law.
(MLn)aq ⇌ (MLn)org
Interaction in organic phase:
After crossing the phase boundary, the species may undergo polymerization, dissociation etc in the organic phase. This can be analyzed either in the same solvent or by re-extracting into aqueous solvent.
Types of extraction techniques
For extraction of the solute from one phase to other, different methods are used. The most generally used techniques are discussed below.
Batch extraction:
In this method the total volumes of organic and aqueous solvents are brought into contact with each other in single batch. This type of extraction is carried out in separatory funnel as shown in fig. The two liquids are put in contact with each other and shaken for sufficient time. After complete extraction, the lower layer is removed and then the upper layer is taken out.
Back extraction or stripping:
Most of the times, the analysis is not carried out in organic phase. On the contrary, the species is brought back into aqueous phase. This can be achieved either by setting appropriate pH or by using another complexing agent to form a complex that is more soluble in water.
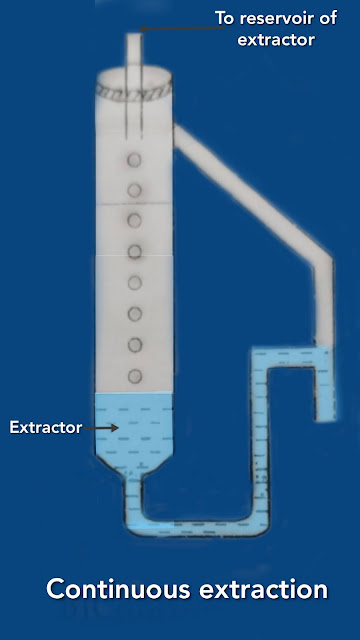
Continuous extraction:
For complete extraction, it is necessary to have a large number of extractions. This is possible using continuous extraction process. For this, the extractant is added dropwise and removed dropwise from the solution of species as shown in Fig.
Counter-current extraction:
In this technique, two immiscible solvents are made to flow in opposite direction in contact with other. This technique is probably the most efficient among all.
Soxhlet extraction:
This method is based on continuous extraction of desired constituent from a solid sample into an organic solvent. It is carried out in special type of apparatus called as soxhlet extractor. With this equipment, extraction can be continued up to 8 to 10 hours without break. Figure shows soxhlet extractor assembly.
Factors affecting extraction efficiency
In this process of extraction, three components are involved, two solvents and one solute. Nature of all three components affects efficiency of the method.
1. Nature of solute species:
Most of the times, it is the metal ion being separated using solvent extraction. It is converted into suitable chelate by adding ligand solution. Due to formation of chelate, the solubility of the species increases in organic solvent. Thus, extraction becomes possible. Hence any factor that enhances the stability of the chelate, increases efficiency of extraction. Such factors are-
Higher basic strength of chelating groups.
Soft-base nature of the donating groups in ligands.
Five or six membered ring formation in chelate that reduces ring strain.
Resonating structures of the chelate.
2. Nature of solvents:
Out of the two solvents, one is generally water. Other solvent is called as extractant. The choice of solvent for extraction is governed by following considerations:
High distribution ratio of the solute and low distribution ratio of undesirable impurities in extracting solvent.
Low solubility in aqueous phase.
Low viscosity and large density difference from the aqueous phase.
Low toxicity and flammability.
Easy recovery of solute for further analysis.
3. Conditions of extraction:
Various conditions adjusted during extraction of one or more components in a mixture affect efficiency of extraction. One of the conditions is pH. By proper adjustment of pH, selective extraction of desired component can be achieved. pH is controlled by using buffers.
e.g. Dithizone can be used as extracting agent for selective extraction of various metals in chloroform under pH conditions as-
Metal ion Optimum pH
Cu(II) 1
Hg(II) 1-2
Ag(II) 1-2
Sn(II) 6-9
Co(II) 7-9
Ni(II) 8
Zn(II) 8.5-11
Pb(II) 8.5-11
4. Synergistic effect:
In some cases, when a mixture of reagents is used for extraction, the extraction efficiency is increased. Such effect is called as synergistic effect. A mixed Complex is formed in this case of dithizone in combination with pyridine or 1,10-phenanthroline.
e.g. Complex of Ni(II) with Dithizone in the presence of 1,10-phenanthroline get extracted according to equation-
Ni² + 2H₂Dz + phen → [Ni(HDz)₂ (phen)] + 2H+
5. Number of extractions:
In the same volume of extractant, if large number of extractions are carried out taking small volume of extractant every time, the efficiency of extraction increases.
This has been discussed in details.
Let us assume that one of the two phases is water and extracting phase is organic phase. The ratio of molar concentrations of solute in two solvents is called as distribution ratio (D) given by-
D=Co
Cw
Co= concentration of solute in organic phase
Cw= concentration of solute in water
Let V= total volume of solution from which solute is to be extracted (generally water)
v= portion of extractant taken every time for extraction (ml)
n= number of extractions
X₀= total weiɡht of solute in water(ɡ)
xn= weiɡht of solute remaininɡ after 'n' extraction in water(ɡ),then
n
Xn= x₀[V/V+Dv]
It should be noted that by increasing the number of extractions and decreasing the column taken for each extraction, one can decrease the amount of unextracted solute, thereby increasing the efficiency of extraction. Efficiency of extraction can be determining percentage of solute extracted.
It is given by-
Percentage efficiency= X₀-Xn ×100
X₀
Applications
Determination of iron using 8-hydroxyquinoline :
Fe(II) can be extracted from aqueous solution with 1% of 8-hydroxyquinoline in chloroform. The Complex is extracted quantitatively in chloroform. It is a dark-coloured Complex that absorbs at 470 nm. It is analyzed spectrophotometrically.
Determination of lead using dithizone:
Lead can be analyzed at very low concentration level using dithizone reagent. Solvent extraction is carried out in chloroform. The method is highly sensitive.
Determination of nickel using dimethylglyoxime (DMG):
Nickel forms a red Complex with DGM that can be extracted in chloroform. The yellow Complex absorbs at 366 nm and 465 nm. Hence it can be estimated spectrophotometrically.
Determination of copper using diethyldithiocarbamate :
Sodium salt of diethyldithiocarbamate (Na-DDC) reacts with copper in weakly acidic or ammoniacal solution to produce a brown colloidal suspension. It may be extracted with an organic solvent like chloroform, carbon tetrachloride etc. Analysis is carried out spectrophotometrically at 435 nm.
Separation of lanthanides:
Similar to ion exchange technique, solvent extraction is also widely used method for separation of lanthanides. For separation of lanthanides, they are extracted with tri-n-butyl phosphate (TBP) in the presence of nitrate ions. Extraction is carried out in continuous countercurrent apparatus.
Separation of Fe³+ and Mg²+ :
Mixture of Fe³+ and Mg²+ is shaken with ethyl acetate as an extractant in strongly acidic conditions. Fe³+ gets selectively extracted in ethyl acetate leaving behind Mg²+. The aqueous phase containing Mg²+ is evaporated to concentrate Mg²+. It is neutralized and pH is adjusted to 10 using ammonia buffer and determined complexometrically using FBT as an indicator. The ethyl acetate solution containing Fe³+ is shaken with water to re-extract iron in aqueous phase. The Fe³+ is reduced to Fe²+ and estimated by redox titration with K₂Cr₂O₇.













Great Blog👍👍
ReplyDeletevery well
ReplyDelete